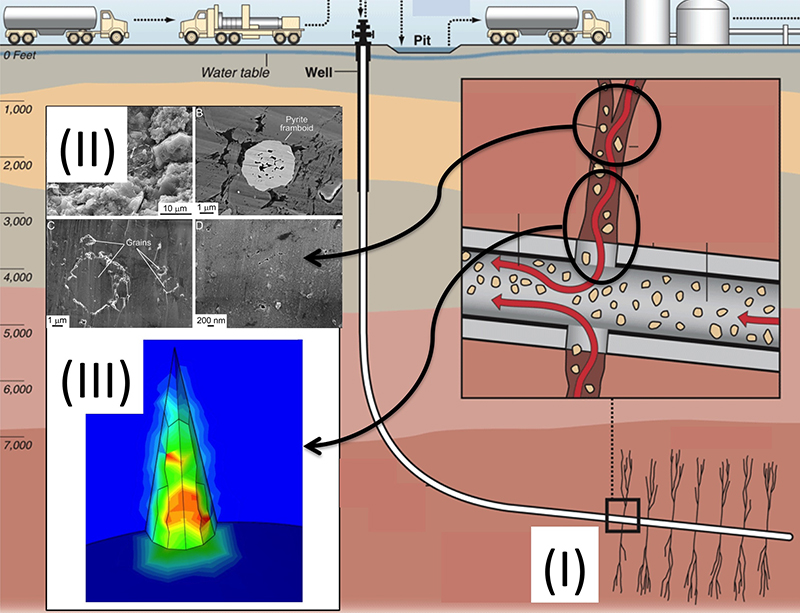
A model system of hydraulic fracturing hazards - fracture pattern formation and multiphase fluid flow. (I) Fracture initiation, propagation, bifurcation (sketch from [Leggett, 2013]). (II) Hydrocarbon extraction from nanometer-scale pores (photos from [Loucks et al., 2009]). (III) Interaction between fractures and the porous rock matrix (figure from [Xu et al., 2014]). (Illustration Courtesy of Chloe Arson.)
Engineering and public policy researchers will pilot a new Ethics Laboratory at Georgia Tech by evaluating the impacts of hydraulic fracturing in Pennsylvania. And they’ll get started this summer with a grant from the Georgia Tech Fund for Innovation in Research and Education (GT FIRE).
School of Civil and Environmental Engineering (CEE) professors Chloe Arson and Lauren Stewart are joining Robert Kirkman from the School of Public Policy on the project.
They want to engage students, faculty and professionals to identify the environmental and ethical risks of hydraulic fracturing and then initiate a public deliberation to define which of those are acceptable risks. The researchers will compare options to address the risks, recommend regulatory efforts to decrease the probability of harm, and debate possible alternatives to hydraulic fracturing.
“We can serve as a link between scientific experts and the [broader] population by analyzing the economical, environmental, societal and ethical facts,” said Arson, who’s leading the effort. “We hope this initiative can help decision makers opt for appropriate energy choices for the economy and for society.”
Arson said hydraulic fracturing will be just the first case study analyzed under the new Ethics Lab. The researchers will seek further funding to consider the implications of other energy geotechnology problems, such as underground storage.
“Scientific, technological and ethical research in this area is needed, and we are willing to collaborate with energy companies, federal agencies and academic experts to propose a systematic view of the energy cycle,” Arson said.
The year-long project will be multidisciplinary, looking at rock mechanics and numerical geomechanics, modeling explosions and blast, and evaluating ethics and policy. The project will focus on the site of Dunkard Township in Pennsylvania, where an explosion recently garnered public attention.
Pennsylvania is one of three main areas where shale gas is being extracted in the United States (the others are in Texas and Louisiana). Together, they provide 18.5 percent of all the natural gas produced in the United States.
Meanwhile, production of shale gas from U.S. wells is growing rapidly. The International Energy Agency reports shale production is projected to increase from 23 percent of total U.S. gas production in 2010 to 49 percent by 2035.
How Hydraulic Fracturing Works
Until the 1940s, shale wells were stimulated by detonating explosives in the rock formation. Then oil and gas companies turned to hydraulic fracturing, which consists of injecting fluids into the rock mass under high pressure to open naturally existing fractures.
Water is mixed with polymers and nitrogen bubbles to create the fracture. Afterward, propping agents are introduced to keep the fractures open once the fluid is withdrawn. That allows for extraction of the natural gas.
Environmental Concerns
Ninety percent of the fluid used to open the rock is water, according to the U.S. Environmental Protection Agency, creating concerns that hydraulic fracturing might limit the amount of water—particularly drinkable water—available for other uses.
There is also increasing concern about water pollution caused by an accidental fluid release from the wells, fluids leaking into aquifers through man-made fractures that intersect with natural faults, and potential leakage from the tanks containing wastewater.
The natural-gas wells also emit volatile organic compounds, which contribute to the formation of smog, and they produce significant amounts of methane, a greenhouse gas about 20 times more potent than carbon dioxide. And methane can induce violent explosions that pose a significant danger to workers and to the public in the surrounding areas. Such explosions at different sites in the United States also have caused secondary explosions at nearby wells and generated large fires.
